
|
Personal Information
More >>特聘研究员A岗 Supervisor of Doctorate Candidates Supervisor of Master's Candidates
Profile
林禄清 博士 博士生导师
联系地址:凌水主校区(化学楼)118-B 邮箱: linluqing@dlut.edu.cn
2013年毕业于日本东京大学, 师从Motomu Kanai 教授,从事铜催化的不对称多手性中心多醇化合物的合成。随后分别在合作导师瑞士日内瓦大学Clement Mazet教授,北海道大学Matsunaga Shigeki教授组里从事金属催化异构化和不对称碳氢键活化反应。在海外学习与工作期间分别获得JST-Erato项目助理研究员奖学金,上原纪念财团奖学金,国家优秀自费留学生奖学金,日本学术振兴会(JSPS)特聘研究员奖学金等资助。相关的研究发表在J. Am. Chem. Soc., Angew. Chem. Int. Ed. 等国际期刊上。
2019年4月加入大连理工学从事基础科研教学工作。
目前已经主持“兴辽英才计划”青年拔尖人才项目,国家自然基金等项目。
课题组主页:https://www.x-mol.com/groups/lin_luqing
课题组常年招收博士后,硕士博士研究生,欢迎感兴趣的学生联系。
论文成果:
23. Spirobipyridine Ligand Enabled Iridium-Catalyzed Site-Selective C-H Activation via Non-Covalent Interactions
Dong,K; Wu, T; Wang, M.*; Lin, L.* Angew. Chem., Int. Ed. 2024, e202411158. https://doi.org/10.1002/anie.202411158

22.In situ copper photocatalysts triggering halide atom transfer of unactivated alkyl halides for general C(sp3)-N couplings
Luo, H; Yang, Y; Fu, Y; Yu, F; Gao, L; Ma, Y; Yang, L*; Wu, K*; Lin, L*. Nat. Commun. 2024, 15, 5647.

21. Silylacylation of Alkenes through N‑Heterocyclic Carbene Catalysis
Xu, H; Zheng, W.; Liu W.-D.; Zhou, Y.*; Lin, L.; Zhao, J.* Org. Lett. 2023, 25, 5579−5584.
20. Visible-Light-Activated Nickel Thiolates for C−S Couplings
Wang, G.; Gao, L.; Feng, Y.; Lin, L.* Org. Lett. 2023, 25, DOI: 10.1021/acs.orglett.3c01474
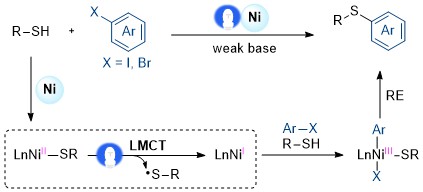
19. Photoinduced Homolysis of the Ni−P Bond via Ligand to Metal Charge Transfer for C−P Bond Formation in Nickel Catalysis
Ma, Y.; Luo, H.; Lin, L.* Org. Lett. 2023, 25, 3492-3496.
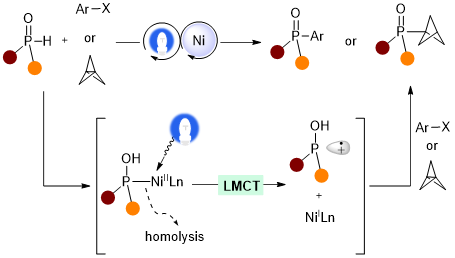
18. Recent Advance in Single Nickel Photocatalysis for Carbon-Heteroatom Bond Formation (Invited Review)
Luo, H.; Feng, Y.; Lin, L.* ChemCatChem 2023, e202300303
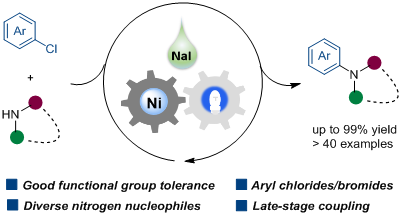
17. Sodium-Iodide-Promoted Nickel-Catalyzed C-N Cross-Coupling of Aryl Chlorides and N-nucleophiles under Visible-Light Irradiation
Feng, Y.; Luo, H.; Yu, F.; Liao, Q.; Lin, L.* Green Chem. 2023, 25, 2361-23614.
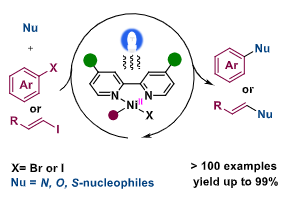
16. Photo-Induced Nickel-Catalyzed Carbon-Heteroatom Coupling
Luo, H.; Wang, G.; Feng, Y.; Zheng, W.; Kong, L.; Ma, Y.; Matsunaga, S.*; Lin, L.* Chem. Eur. J. 2023, e202202385. (Highlighted by ChemistryViews)
15. Light-Promoted Arylsilylation of Alkenes with Hydrosilanes
Zheng, W.; Xu, Y.; Luo, H.; Feng, Y.; Zhang, J.; Lin, L.* Org. Lett. 2022, 24, 7145–7150.
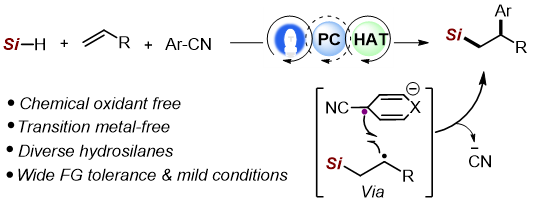
14. Light-Promoted Nickel-Catalyzed Aromatic Halogen Exchange
Feng, Y.; Luo, H.; Zheng, W.; Matsunaga, S.; Lin, L.* ACS Catalysis 2022, 12, 11089−11096.
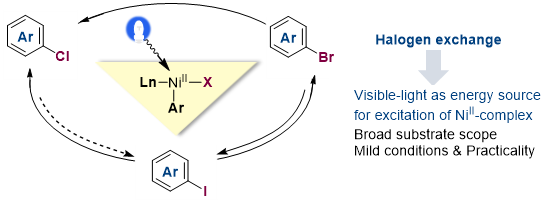
13. Nickel-Catalyzed Thioesterification Enabled by a Visible-Light Organophotoredox Catalyst under Mild Conditions
Zheng, W.; Xu. Y.; Lin, L.* ChemPhotoChem 2022, 6, https://doi.org/10.1002/cptc.202100264
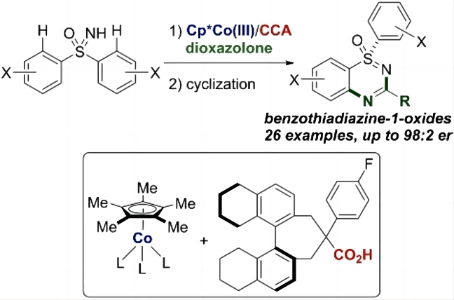
12. Cobalt(III)/Chiral Carboxylic Acid-Catalyzed Enantioselective Synthesis of Benzothiadiazine-1-Oxides via C–H Activation
Hirata, Y.; Sekine, D.; Kato, Y.; Lin, L.; Kojima, M.; Yoshino, T.*; Matsunaga, S.* Angew. Chem., Int. Ed. 2022, https://doi.org/10.1002/anie.202205341
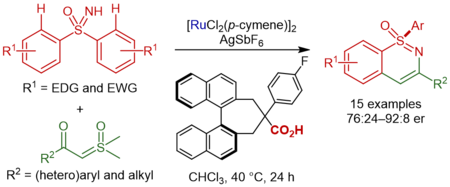
11. Ru(II)/Chiral Carboxylic Acid-Catalyzed Enantioselective C–H Functionalization of Sulfoximines
Huang, L.-T.; Hirata, Y.; Kato, Y.; Lin, L.; Kojima, M.; Yoshino, T.*; Matsunaga, S.*Synthesis 2021, 53, DOI: 10.1055/a-1588-0072
10. Development of Pseudo-C2-symmetric Chiral Binaphthyl Monocarboxylic Acids for Enantioselective C(sp3)–H Functionalization Reactions under Rh(III) Catalysis
Kato,Y.; Lin, L*; Kojima, M.; Yoshino, T.*; Matsunaga S.* ACS Catalysis 2021, 11, 4271−4277. https://doi.org/10.1021/acscatal.1c00765
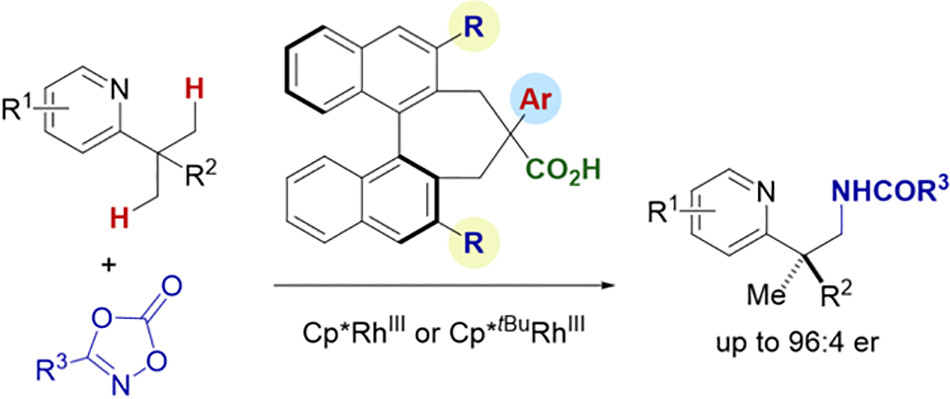
9. Photocatalytic Generation of π-allyltitanium Complexes via Radical intermediates
Li, F.; Lin,S.; Chen,Y.; Shi, C.; Yan, H.; Li, C.; Wu, C.; Lin, L.; Duan, C.; Shi, L.* Angew. Chem. Int. Ed. 2021, 60, 1561-1566.

8. Cp2TiIIICl Catalysis in a New Light
Chen, Y.; Lin, S.; Li, F.; Zhang, X.; Lin, L.; Shi, L.* ChemPhotoChem 2020, 4, 659–663.
Before DLUT
7. Chiral Carboxylic Acid‐Enabled Achiral Rhodium(III)-Catalyzed Enantioselective C−H Functionalization
Lin, L*; Fukagawa, S.; Sekine, D.; Tomita, E.; Yoshino, T.*; Matsunaga S.* Angew. Chem. Int. Ed. 2018. 57, 12048-12052. (SYNFACT) https://doi.org/10.1002/anie.201807610
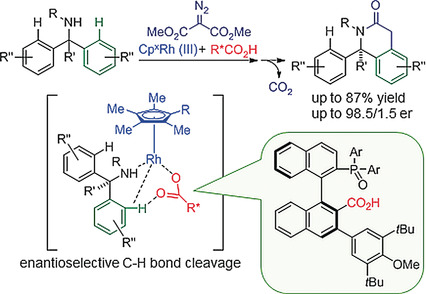
6. Palladium-Catalyzed Long-Range Deconjugative Isomerization of Highly Substituted α,β-Unsaturated Carbonyl Compounds
Lin, L.; Romano, C.; Mazet, C. * J. Am. Chem. Soc. 2016, 138, 10344−10350. https://doi.org/10.1021/jacs.6b06390
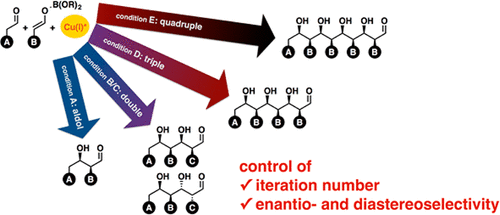
5. Catalytic Asymmetric Iterative/Domino Aldehyde Cross-Aldol Reactions for the Rapid and Flexible Synthesis of 1,3-Polyols.
Lin, L.; Yamamoto, K.; Mitsunuma, H.; Kanzaki, Y.; Matsunaga, S. * Kanai, M. * J. Am. Chem. Soc. 2015, 137, 15418–15421. (SYNFACT of the month) https://doi.org/10.1021/jacs.5b11192
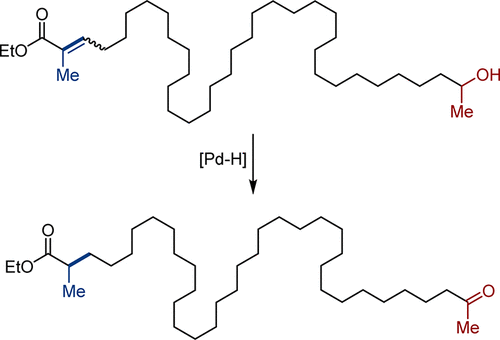
4. Scope and mechanism in palladium-catalyzed isomerizations of highly substituted allylic, homoallylic, and alkenyl alcohols.
Larionov, E.; Lin, L.; Guénée, L.; Mazet, C.* J. Am. Chem. Soc. 2014, 136, 16882–16994. https://doi.org/10.1021/ja508736u

3. Rh-catalyzed aldehyde-aldehyde cross-aldol reaction under base-free conditions: in situ aldehyde-derived enolate formation through orthogonal activation.
Lin, L.; Yamamoto, K.: Matsunaga, S. *; Kanai, M. * Chem. Asian J. 2013, 8, 2974-2983. (Selected as a Frontispiece) https://doi.org/10.1002/asia.201300928
2. Rhodium-catalyzed cross-aldol reaction: In situ aldehyde-enolate formation from allyloxyboranes and primary allylic alcohols
Lin, L.; Yamamoto, K.: Matsunaga, S.; Kanai, M. * Angew. Chem. Int. Ed. 2012, 51, 10275–10279. (SYNFACT) https://doi.org/10.1002/anie.201205680
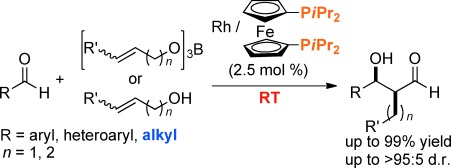
1. Catalytic asymmetric Ring-Opening of meso-Aziridines with malonates under heterodinuclear rare earth metal schiff base catalysis
Xu, Y.; Lin,L.; Kanai, M.; Matsunaga, S. *; Shibasaki, M. * J. Am. Chem. Soc. 2011, 133, 5791–5793. https://pubs.acs.org/doi/10.1021/ja201492x
